R&D biosimilar expertise

The Fresenius Kabi Biosimilars state-of-the art research and development centre is based in Eysins, Switzerland. Its current focus is on the areas of autoimmune diseases and oncology. The centre was created in order to strengthen our biosimilars pipeline, thus enabling access to biosimilar treatment options for patients worldwide.
Please watch our R&D Lab video here:
Our centre’s expertise includes the following areas:
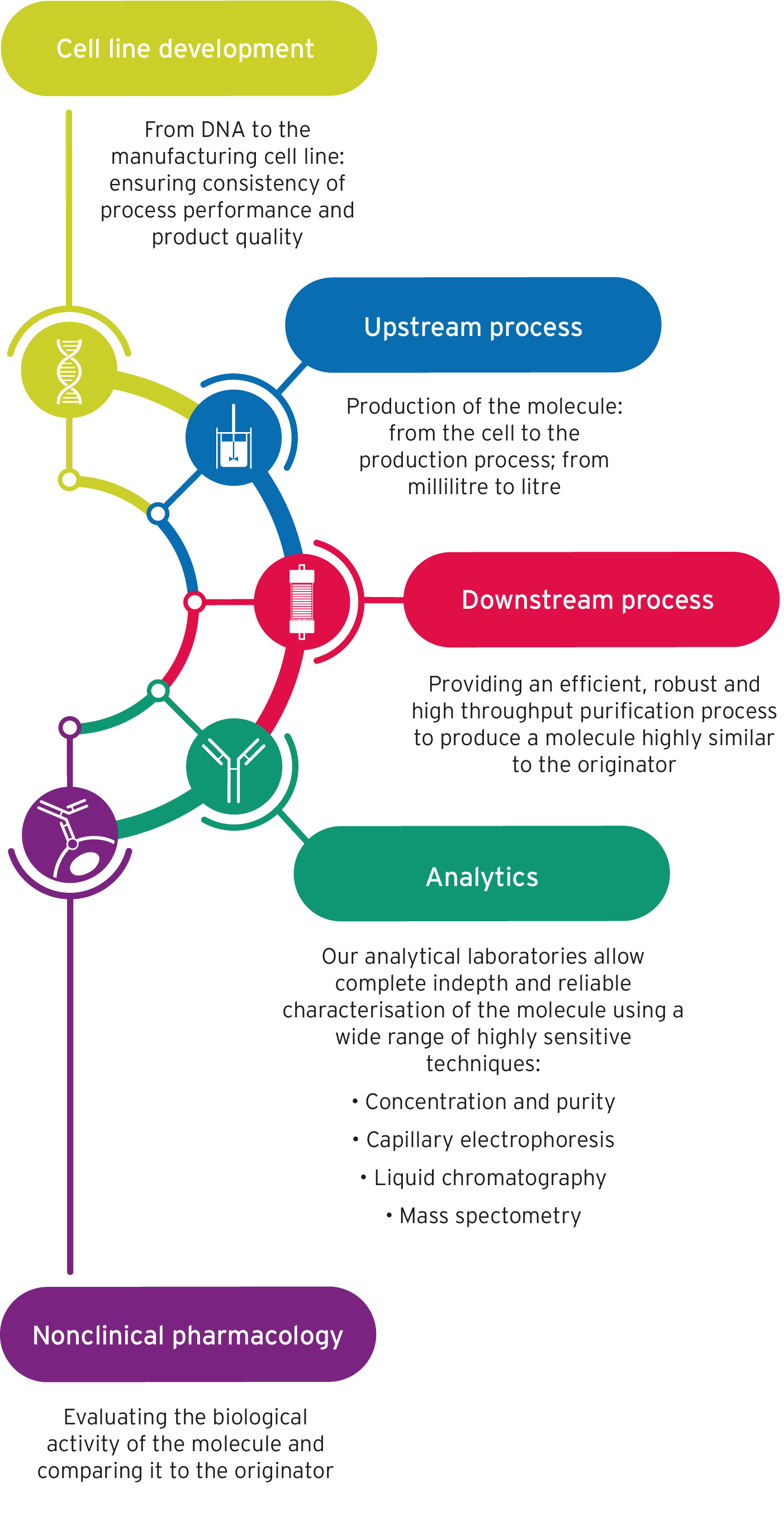
- Cell line development – from DNA to the manufacturing cell line: ensuring consistency of process performance and product quality
- Upstream process – production of the molecule: from the cell to the production process; from millilitre to litre
- Downstream process – providing an efficient, robust and high throughput purification process to produce a molecule highly similar to the originator
- Analytics – our analytical laboratories allow complete indepth and reliable characterisation of the molecule using a wide range of highly sensitive techniques:
- Concentration and purity
- Capillary electrophoresis
- Liquid chromatography
- Mass spectometry
- Nonclinical pharmacology – evaluating the biological activity of the molecule and comparing it to the originator
Our areas of focus, when creating a Biosimilar product that is highly similar to the originator product are:
- Characterisation
- High throughput
- Quality screening
- Reliability
- Comparability
